Research
There are several exciting research projects in the Chemistry Department! Chemistry majors and minors have the opportunity to work with faculty on these projects and present their findings at state and national meetings. You can find more information about on-campus summer research opportunities here.
If you are interested in conducting research at another institution during the summer,
please follow our departmental social media for opportunities and application dates.
You can find us on Facebook @OBUChemistry and on Instagram @ouachitachemistry.
Joe Bradshaw
Porphyrins and Quantum Dots as Photodynamic Therapy Agents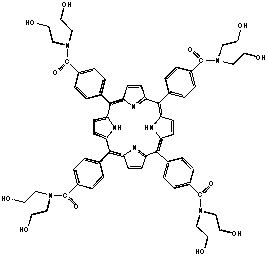
There are several different projects that students have been investigating. The first project involves the synthesis and characterization of porphyrins and their coupling with materials to make them water-soluble. Porphyrins have fluorescent properties, and some derivatives have been used in Photodynamic Therapy (PDT) a novel treatment of cancer. Metalloporphyrins have been shown to be tumor specific and thus have potential as possible tumor therapy agents. It has been recently demonstrated that the synthesis of these neutral porphyrins containing poly-alcohol functionalities to achieve water-solubility can be carried out. This project examines the synthesis, purification and characterization of these novel water-soluble porphyrins and their cytotoxic properties.
The second project entails the synthesis, characterization, and measuring the relaxivity of target-specific MRI (magnetic resonance imaging) contrast agents. Although MRI contrast media have been available for some time, designing MRI contrast media that is organ specific has been difficult, particularly designing contrast agents that cross the blood brain barrier. This project involves the synthesis of a novel gadolinium MRI contrast media for brain imaging.
The third project involves the development of more efficient solar cells for use in long-term space flight. Students will be synthesizing new zinc(II) porphyrin materials (Figure 1) and incorporating TAMRA-azide (Figure 2), a carboxytetramethylrhodamine derivative. TAMRA will be added to the porphyrin core using “click” chemistry. Incorporating this substituent on the periphery of the porphyrin would address the goal of using porphyrins in broad-band light harvesting. After formation, these porphyrins will be characterized spectroscopically (IR, UV-vis, NMR). Finally, the ability of these novel porphyrins to behave as solar efficient materials will be tested.
Sharon K. Hamilton
Developing Biomimetic Polymers Towards Modern Wound Healing Materials
My primary research interest lies in the modification and application of polymers for various biomedical purposes. I am specifically interested in the medical application of modified polymers and my lab utilizes a multi-pronged strategy to our research investigations: (1) developing degradable fibers that exhibit a controlled small molecule release profile; (2) preparing natural polymer-based scaffolds with additives to observe additive impact on drug release profiles; and (3) developing cleavable natural polymer-antibiotic conjugates to promote drug release from electrospun scaffolds; and (4) synthesizing biomimetic polymers as cost-alternative materials for use in biomedical applications.
Recent evolutions in the field of biomaterials have focused on developing materials that can facilely interface with biological systems to treat or replace tissues or functions of the body. Natural polymers including polysaccharides have been investigated as suitable biomaterials to mimic the environment of tissues and facilitate tissue regeneration. Many natural polymers have been successfully electrospun into nanofibers for regenerative medicinal uses including wound healing, tissue engineering, and drug delivery. Modern wound healing treatments have capabilities including preventing infection, encouraging cell growth, and mimicking the extracellular matrix, thanks in part to incorporated biomolecules. However, little research has been published on the development of cost-effective synthetic analogs to the biomolecules that facilitate these capabilities.
My lab is currently working on the development of bioresponsive fiber mats that contain synthetic biomolecule analogs with the long-term goal of generating a better understanding of natural polymer-based wound healing materials and the cellular responses towards these materials for implementation in wound healing treatments. As a step towards this goal, synthetic biomolecule analogs must be synthesized and electrospun to be compared to biomolecule nanofiber scaffolds.
Students in my lab modify polymers via multi-step organic syntheses and follow the process through the purification and analysis of the final products. The biomimetic polymers are then electrospun into fiber mats which undergo further investigation. As projects progress, it is anticipated that students will have the opportunity to develop cell culture skills and take part in animal studies through our collaborator. My students are exposed to a variety of analytical techniques and laboratory equipment and are expected to perform literature searchers, further develop their critical thinking skills, and help outline experimental procedures. This research is currently funded through ;a J. D. Patterson Grant, the Arkansas INBRE program, and the Arkansas Space Grant Consortium.
Tim Hayes
The Role of Retinoblastoma Family Proteins in the Differentiation of Pre-Adipocytes
My research interests center on the molecular mechanisms involved in the permanent exit of cells from the cell cycle as they terminally differentiate. Cells that previously proliferated in response to external signals now respond to those same signals in a different way. What changes in the cell to alter its response?
The major project in my lab looks at this question using the terminal differentiation of pre-adipocytes as a model. Pre-adipocytes proliferate rapidly and can be cultured in the lab. When they are contact inhibited they can be treated with a cocktail of hormones and will respond by differentiating. Over the course of the next few days they express fat cell proteins, produce lipid droplets and turn into adipocytes. During this time many of the cells divide once or twice but when this period is past they are post-mitotic- they never divide again.
My lab is working on the roles of the Retinoblastoma family proteins p107, p130 and pRB during the early stages of this process. We are ‘knocking down’ the expression of each of these proteins individually and in combinations to determine the effect on differentiation. Our experiments utilize cell culture, protein techniques (electrophoresis, Western blotting, etc.), cell biology methods (virus infection, microscopy and cell staining with dyes and antibodies) and molecular biology techniques (transfection, electroporation, making plasmids, PCR, etc.) as required by the particular experiment being done. This research is funded by a J. D. Patterson Grant.
Analysis of bis-phenol A in Water Samples using Fluorescence Spectroscopy
Bis-phenol A (BPA) is used in the production of epoxy resins and polycarbonate plastics and frequently remains in these materials. In vitro and in vivo experiments show that BPA exhibits weak estrogenic activity by binding to and activating estrogen receptors. Suspected effects of modified endocrine functions may be reduced fertility, altered development, and cancer in estrogen-sensitive tissues. Infants and children are particularly at risk due to their still developing neurological and endocrine systems and the fact that their ability to detoxify and eliminate substances such as BPA is immature. This concern led to a change in the plastic used for baby bottles and other containers, which removes BPA from the plastic, allowing manufacturer’s to claim their products are “BPA free”. Despite this reduction of BPA in current food packaging, BPA is still present in our environment due to BPA-containing waste in landfills or ocean trash. The environmental sources, though at low levels, are a particular concern for the exposure of infants and children and also for aquatic organisms.
BPA is a fluorescent compound, which means following excitation, it will emit radiation at a longer wavelength than the exciting wavelength. BPA is excited at ~280 nm and emits and ~310nm. This emitted light can be measured and correlated to the concentration of BPA present in a sample. Fluorescence is a very sensitive and selective technique, which makes it possible to determine very low concentrations BPA with fewer concerns about outside interferences compared to absorption spectroscopy.
The goals of this project are to obtain fluorescence data for BPA and to explore the effects of temperature and time on the leaching of BPA-containing materials into water samples. This research is funded by a J. D. Patterson Grant.
Joe Jeffers
The Life and Works of Frederick Sanger, Nobel Laureate in Chemistry 1958, Nobel Laureate in Chemistry 1980
Dr. Frederick Sanger was awarded the Nobel Prize in Chemistry in 1958 for his work in determining the structure of insulin, the first protein molecule sequenced. He became only the third two-time recipient of the Nobel Prize when he shared the 1980 Nobel Prize in Chemistry for developing techniques for sequencing DNA molecules. Dr. Sanger worked first in the Biochemistry Department at Cambridge University in England. Then he worked at the Medical Research Council Laboratory of Molecular Biology in Cambridge. I have interviewed Dr. Sanger and many of his colleagues and family members. I continue research to prepare articles for the Bulletin for the History of Chemistry and to write a biography of Frederick Sanger. This research is funded by a J. D. Patterson Grant.